Using vanadium catalysts to breakdown biomass to produce chemical feedstocks
The Los Alamos work highlighted in Angewandte Chemie demonstrates that a phenolic lignin model compound may be broken down selectively into useful components using a vanadium catalyst
In a paper published in the prestigious chemistry journal Angewandte Chemie International Edition, Los Alamos scientists Susan Hanson (C Division), Ruilian Wu, and Pete Silks (B Division) describe a significant advance in catalysis science that furthers the important goal of breaking down biomass into high value commodity chemicals. The paper was published in the online Early View February 7th and will be highlighted on the inside cover of the April print edition of the journal.
In light of diminishing fossil petroleum reserves, non-food biomass (lignocellulose), is viewed as a potentially attractive alternative to petroleum as a feedstock for the production of renewable chemicals and fuels. In fact, the DOE estimates that the US could produce as much as 1.3 billion tons per year of lignocellulose, enough to replace 30% of the current US petroleum consumption. However, efficient transformation of lignin, an integral and problematic component of lignocellulose, into useful compounds remains a major challenge. Lignin is a randomized aromatic polymer that is resistant to decomposition, both in nature and in the lab. Efficient breakdown of lignin has been and continues to be a major research goal for scientists pursuing new ways of converting biomass to fuels.
The Los Alamos work highlighted in Angewandte Chemie demonstrates that a phenolic lignin model compound may be broken down selectively into useful components using a vanadium catalyst. Vanadium is an inexpensive, earth-abundant metal that is well suited for promoting oxidations in air. The researchers showed that two different vanadium catalysts can break apart phenolic lignin molecules in different ways, an advance which opens the door to developing selective methods for oxidizing lignin substructures using vanadium.
For more information read the complete article, "C-C or C-O Bond Cleavage in a Phenolic Lignin Model Compound: Selectivity Depends on Vanadium Catalyst." (Angew. Chem. Int. Ed Susan K. Hanson, Ruilian Wu, and Louis A. "Pete" Silks. 2012, 51, DOI: 10.1002/anie.201107020). This work was funded by LDRD.
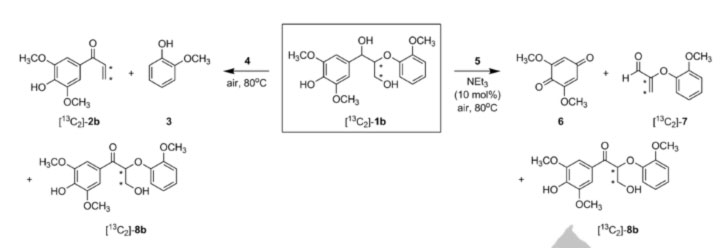
Figure 1. Oxidation of phenolic lignin model compound 1b (center) with vanadium catalysts (4) yields C-O bond cleavage and (5) C-C bond cleavage. This ability to selectively break down lignin in mild conditions is an important step towards turning lignin into useful chemicals or fuels.
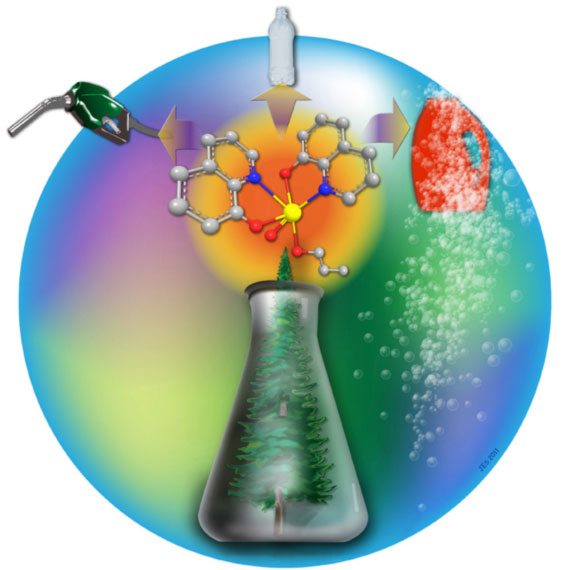
Figure 2. Vanadium complexes catalyze C-C and C-O bond cleavage reactions in lignin model compounds using air as the oxidant. In their communication (DOI: 10.1002/anie.201107020), S. K. Hanson, R. Wu, and L. A. "Pete" Silks report that the selectivity for C-C or C-O bond cleavage depends on the identity of the vanadium catalyst, suggesting the promise of homogeneous catalysts for controlling selectivity in the aerobic oxidation of lignin. (Picture by Josh Smith, LANL Chemistry Division).