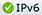STE Highlights, April 2023
Earth and Environmental Sciences
New mass spectrometer offers dynamic capabilities
Researchers overcome Catch-22 of biosensor development to address the plastic waste problem
Explosive Science and Shock Physics
Experiment reveals plasma evolution in multi-species shock waves
Materials Physics and Applications
Overcoming the voltage induced instability problem in perovskite semiconductor detectors
Materials Science and Technology
Novel technique holds potential for developing viable anode-free solid-state batteries
Research sheds light on mechanisms impacting fuel cell durability
Materials-by-design approach reveals lowered work function for lanthanum hexaboride
Earth and Environmental Sciences
New mass spectrometer offers dynamic capabilities
The new isotope Ratio Mass Spec ULTRA, a novel capability to support signature science arenas for energy, climate, and national security.
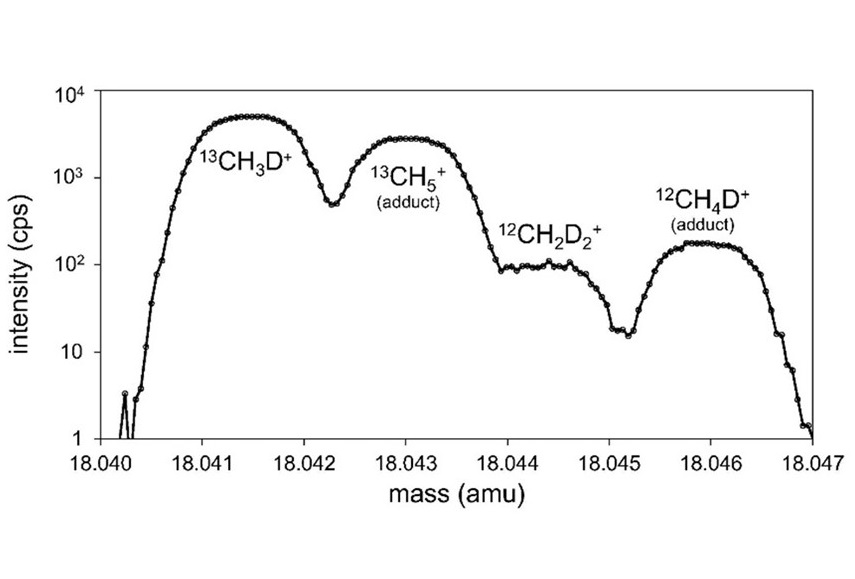
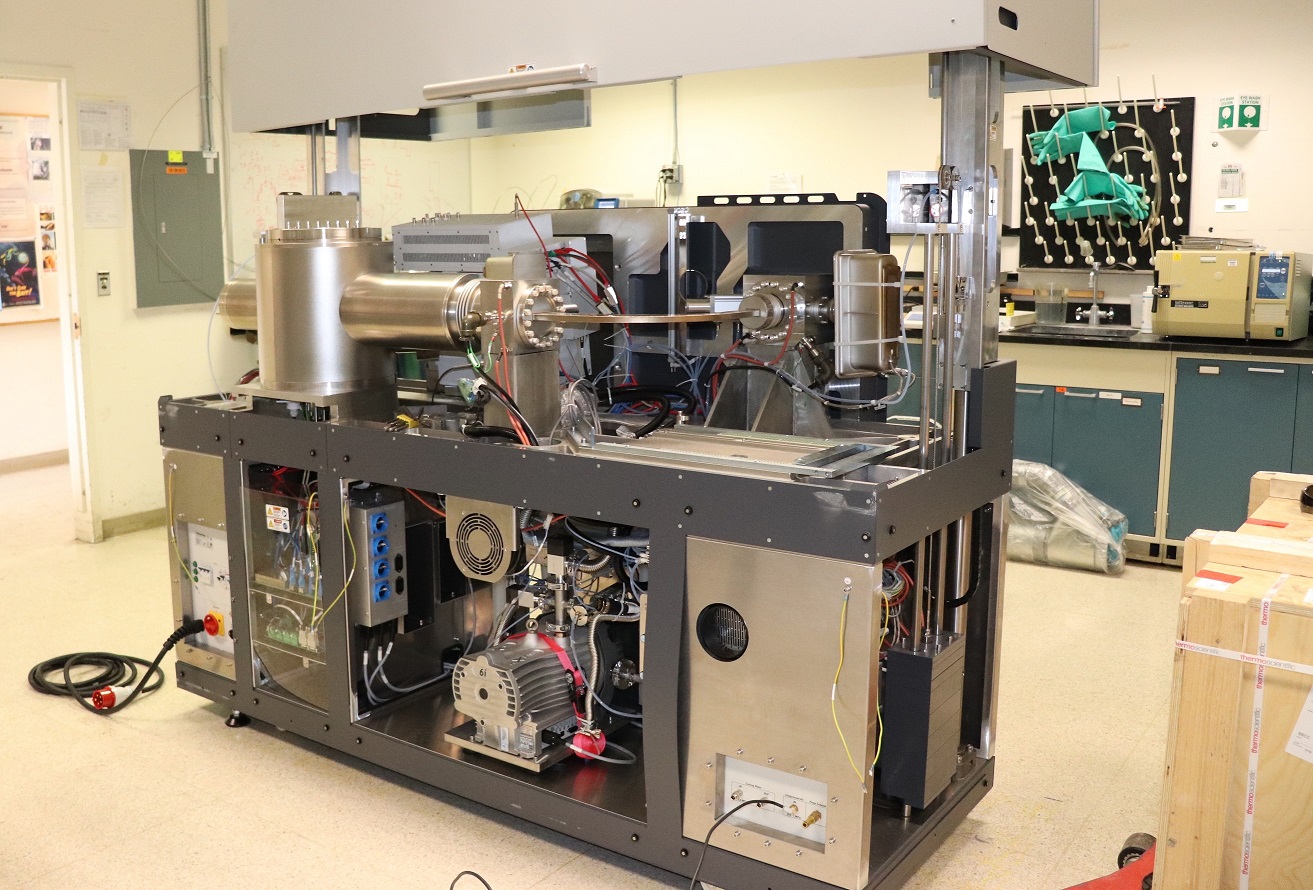
Figure demonstrating the new isotope Ratio Mass Spec ULTRA, a novel capability to support signature science arenas for energy, climate and national security.
The Lab’s Earth and Environmental Sciences (EES) Division recently purchased and installed a powerful gas-source, light-stable isotope mass spectrometer that discriminates critical mission-specific processes. Included with installation were significant facility upgrades to accommodate the new instrument, and additional associated upgrades of EES lab space are ongoing. The ThermoScientific Ultra High Resolution Isotope Ratio Mass Spectrometer supports applications in energy, climate, environment, nonproliferation, treaty verification, forensics, nuclear sciences, high explosives testing and manufacturing, geothermal and repository sciences and subsurface hydrogen and carbon dioxide storage/sequestration. Potential also exists for unprecedented high-resolution analyses of noble gas isotopes and other gases.
This instrument’s resolution is approximately two orders of magnitude greater than any comparable Lab capability, boosting EES’ stable-isotope signature science and placing the division at the DOE forefront in light stable isotope research and in studying unique signatures of interest to agencies including the National Nuclear Security Administration, Department of Energy, Department of Defense and Department of Homeland Security, as well as the intelligence community and industry.
Multiple isotopic substitutions in a single molecule such as carbon dioxide, methane, nitrous oxide, oxygen and hydrogen are cutting-edge signatures for discriminating critical processes. The new mass spectrometer can deliver previously unprecedented stable isotope resolution for a number of gases of interest. The particular specialty of the Ultra instrument is the capability to measure what is colloquially referred to as “clumped” isotopes where there is more than one substitution of the rarer isotope, e.g., 18O18O, 15N15N, 13CH3D & 12CH2D2, DD. In such cases the Ultra can discriminate target molecules from adduct molecules that form in the ion source, eliminating interference with analyses at a given cardinal mass.
The new instrument will support a number of applications for potential sponsors. The spectrometer can conduct identification of unique signatures related to nuclear material processing, transport and weaponization. It can support the identification of hydrogen gas sources and migration pathways in underground nuclear storage facilities and in a future hydrogen economy. The spectrometer can add value in examining volatile copmonents, including volatiles and noble gases, of undeground nuclear explosions. The instrument may also be able to examine signatures related to weapons performance and manufacturing efficiencies.
Mission
The work supports the Global Security mission area and the Complex Natural and Engineered Systems capability pillar.
Technical contact: Thom Rahn (EES-14)
Researchers overcome Catch-22 of biosensor development to address the plastic waste problem
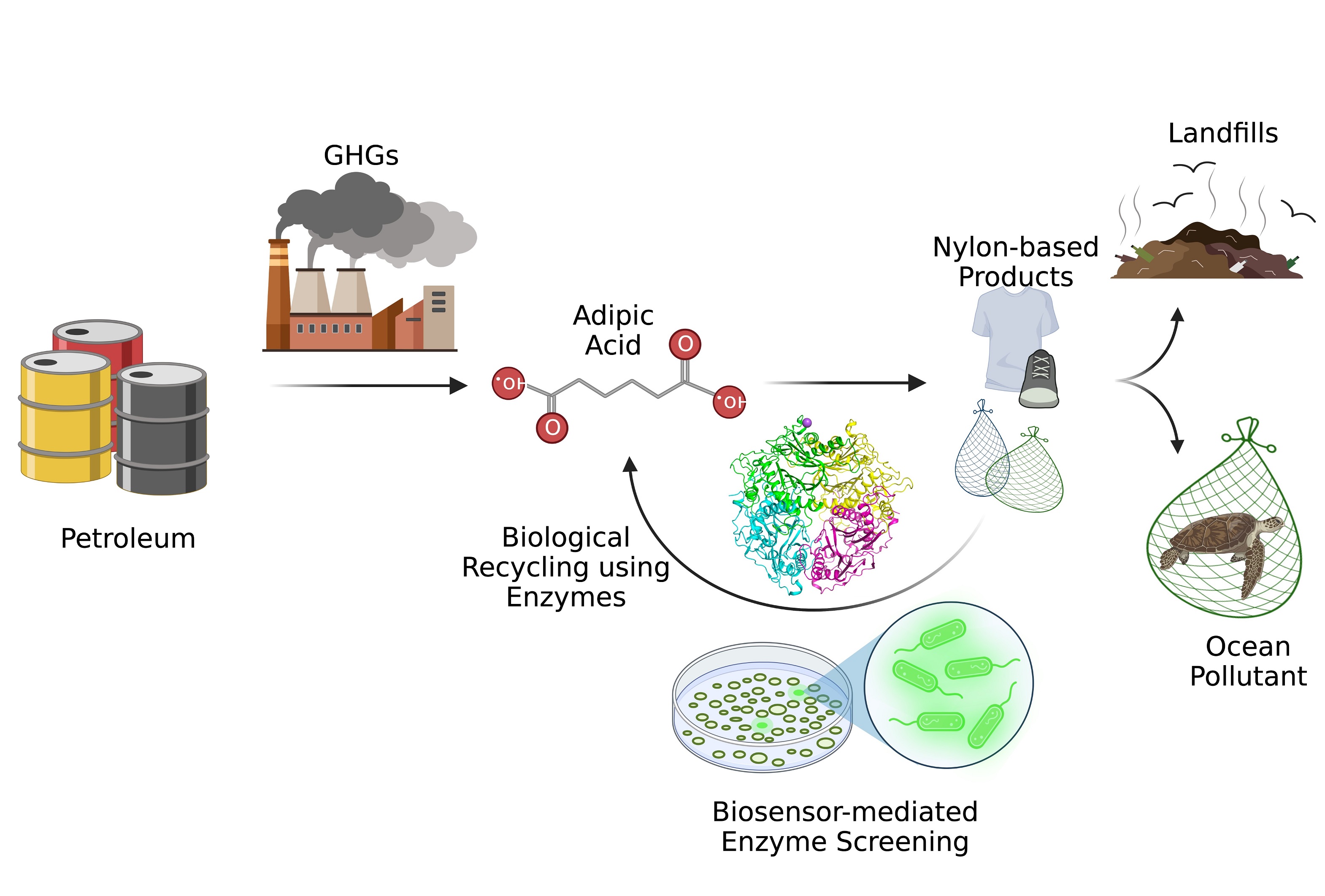
Adipic acid is a central molecule in the chain from petroleum-based manufacturing (and its attendant greenhouse gas emissions) to nylon-based products, which at the end-of-life are discarded in the landfills or remain in oceans as a major pollutant affecting the marine life. The developed biosensor will aid discovery and engineering of novel enzymes for efficient biological recycling of adipic acid, in an effort towards a circular economy.
Researchers in Los Alamos National Laboratory’s Biology division have developed biosensing technology that can help address the problem of plastic waste. As described in the journal ACS Synthetic Biology, the research team engineered a sensitive biosensor for adipic acid (ADA), a polymer building block and product of the degradation of nylon. The biosensor will assist expedited discovery and engineering of biocatalysts — typically enzymes or microbes — that can degrade nylon waste, such as textiles, used carpets and fishing nets. The tool may help clean the environment and also aid in the recovery of the chemical building blocks from such nylon waste as part of biological recycling efforts.
The project tackles a “Catch-22” of a whole cell biosensor development stemming from the requirement that anthropogenic molecules need both a custom transporter and a transcription factor. In order to confirm the transporter activity, one needs a biosensor, but establishing a biosensor requires a functional transporter in a microbial host. For the development of the ADA biosensor, the team leveraged their previous invention, a cis,cis-muconate (ccMA) sensor in Pseudomonas putida bacterium, to rapidly optimize the functioning of a transporter such as MucK, which has been shown to transport various dicarboxylic acids, and can transport ADA. The team then used the stable MucK transporter to evolve a transcription factor PcaR (natively activated by beta-ketoadipic acid, BKA), from P. putida, for the anthropogenic molecule ADA.
Transcription factors in general are highly specific, so even though there is only a small difference in the native inducer (BKA) and the anthropogenic molecule (ADA), native PcaR failed to respond to the new molecule. Consequently, the team used computational modeling expertise, focused mutational library design and high throughput screening coupled to flow cytometry to create and screen 20 million variants of PcaR for response towards ADA. Three variants of PcaR were finally isolated that responded to various degrees to ADA; these functional variants were rare and only one in a million, highlighting the “needle-in-a-haystack” problem in protein engineering.
The team achieved stable activity for the MucK transporter for over 70 generations. They also realized a specificity switch in the engineered PcaR by over 35-fold in favor ADA over BKA at low concentrations. The ADA and BKA biosensors demonstrated high sensitivity and sensor response: detection in concentrations of less than 10 micromoles and an approximately 50-fold change in fluorescence response in P. putida, respectively. The BKA biosensor will aid optimization of microbial strains for synthesis of BKA, which is also a high-value polymer precursor. The ADA biosensor is the first-ever biosensor in P. putida and will help discovery and engineering of efficient biocatalysts for the deconstruction of nylon waste and recycling of building blocks that constitute nylon.
Funding and mission
This work was supported by Department of Energy’s Office of Energy Efficiency and Renewable Energy, Bio Energy Technologies Office (BETO), as well as the Advanced Materials and Manufacturing Technologies Office (AMMTO). The work supports the Energy Security mission area and the Complex Natural and Engineered Systems capability pillar.
Reference
“Tackling the Catch-22 Situation of Optimizing a Sensor and a Transporter System in a Whole-Cell Microbial Biosensor Design for an Anthropogenic Small Molecule,” ACS Synthetic Biology, 11, 12, 39996 (2022); DOI: 10.1021/acssynbio.2c00364. Authors: Sang-Min Shin, Ramesh K. Jha and Taraka Dale (Los Alamos National Laboratory).
Technical contacts: Ramesh Jha (B-IOME), Taraka Dale (B-IOME)
Explosive Science and Shock Physics
Experiment reveals plasma evolution in multi-species shock waves
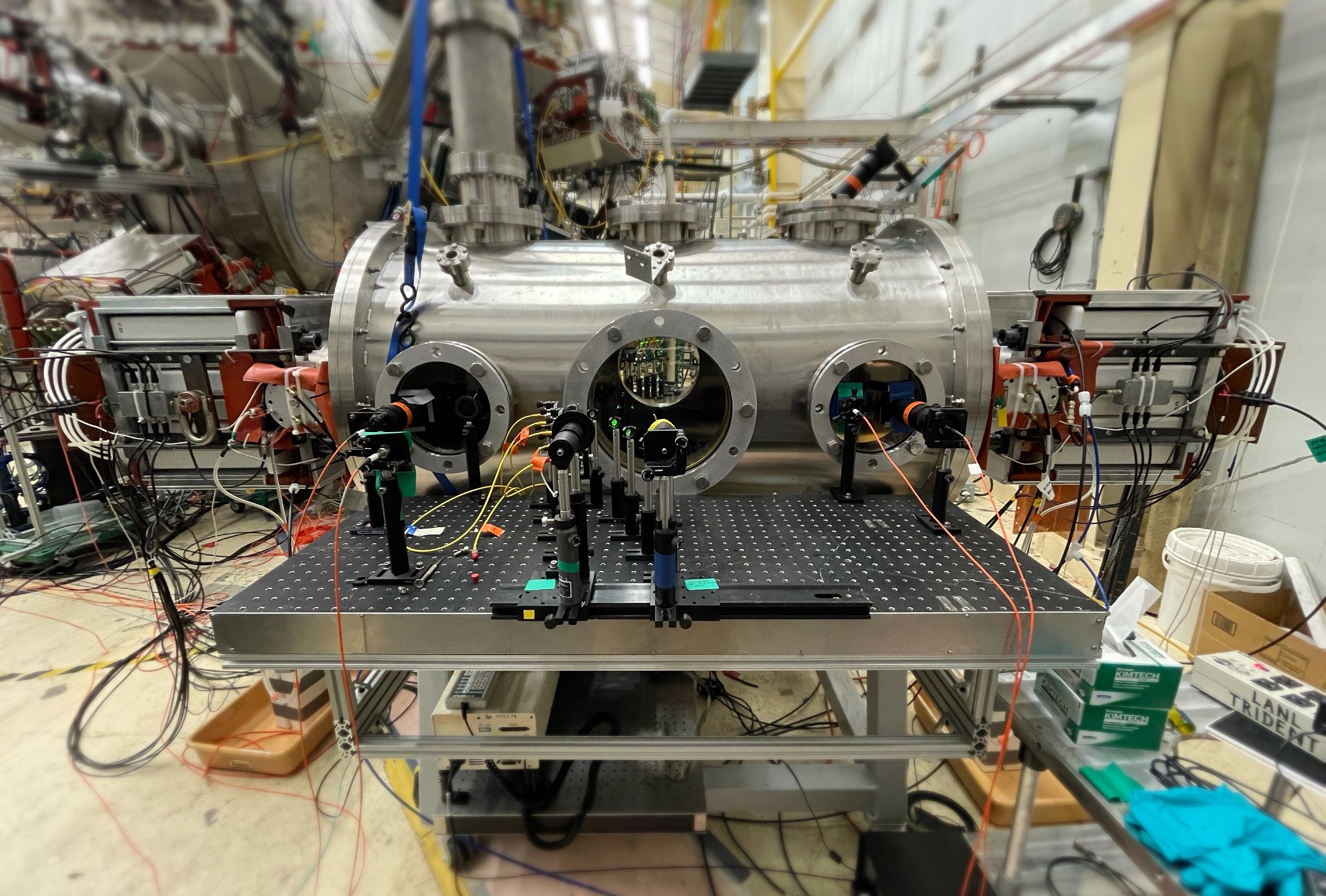
Setup in Physics division labs for the multi-ion-species plasma shock experiment. Multi-ion-species plasma shocks are created by colliding two plasma jets head-on in a 76-cm diameter cylindrical vacuum chamber, with laser and optical diagnostics fielded on the chamber viewports.
Fast camera images of shock formation at 50 and 57 microseconds, respectively, relative to the moment when the capacitor banks in the guns discharge. A microsecond is equal to one millionth (0.000001) of a second.
In inertial confinement fusion (ICF), a fusion fuel consisting of a one-to-one mixture of deuterium and tritium atoms is compressed by strong shock waves driven by laser beams or X-rays to achieve thermonuclear burn conditions. Understanding the evolution of plasma density, temperature and species concentration during these multi-species shock transits is a highly challenging aspect of computational modeling and prediction of ICF ignition. In a paper recently published in Physical Review Letters, a research team led by Los Alamos National Laboratory scientists was able to employ the collision of supersonic plasma jets to create and diagnose a multispecies plasma shock containing two ion species, lending new insight into these physics.
Compared to laser-driven implosions, the plasma-jet driven experiment created the collisional plasma shock interaction at a lower density, larger length scale and slower time evolution (microseconds rather than nanoseconds), allowing experimental measurements to be obtained with improved spatial and temporal resolution. The team’s results reveal for the first time how two separate ion species (argon and nitrogen) evolve with time in the plasma shock. Analysis of the experimental data allowed for the determination of the overall effective ion diffusion coefficient, and thereby enabled an improved validation of the fundamental interspecies transport theory explaining the plasma evolution driven by the shock. The team also observed significant ion heating and temperature separation during shock formation. These signatures provide new data valuable for advancements in modeling HED (high-energy-density) and ICF experiments. The problem was simulated by scientists in the lab's XCP-6 computational physics division, using the iFP (Ion Fokker-Planck) kinetic simulation code, finding qualitative agreement with the experimental results.
The experiment was carried out at the Physics division laboratories at Los Alamos National Laboratory. The team used plasma guns from the 36-gun Plasma Liner Experiment (PLX), an ARPA-E funded project to demonstrate the feasibility of an innovative alternative fusion energy approach known as plasma-jet-driven magneto-inertial fusion (PJMIF). The development of the advanced plasma guns at PLX, carried out in a multi-institutional collaboration including collaborators at HyperJet Fusion Corporation, University of Alabama in Huntsville, and University of New Mexico, made available the unique, longer-lived plasma shock critical to capturing the measurements.
Funding and mission
The research was supported by the Laboratory Directed Research and Development Early Career Research program at Los Alamos National Laboratory. The plasma guns used in this work were designed and built by HyperJet Fusion Corporation for the Plasma Liner Experiment (PLX) collaboration, under funding support from the Advanced Research Projects Agency-Energy (ARPA-E) of the U.S. Department of Energy, coordinated at LANL by the Science Program Office of Applied Energy (SPO-AE). The work supports the Global Security mission area and the Nuclear and Particle Futures capability pillar.
Reference
“Experimental Measurements of Ion Diffusion Coefficients and Heating in a Multi-Ion-Species Plasma Shock,” Physical Review Letters, 130, 145101 (2023); DOI: 10.1103/PhysRevLett.130.145101. Authors: F. Chu, B. D. Keenan and S. J. Langendorf (Los Alamos National Laboratory); A. L. LaJoie, L. Webster and M. A. Gilmore (University of New Mexico).
Technical contact: Feng Chu (P-4)
Materials Physics and Applications
Overcoming the voltage induced instability problem in perovskite semiconductor detectors
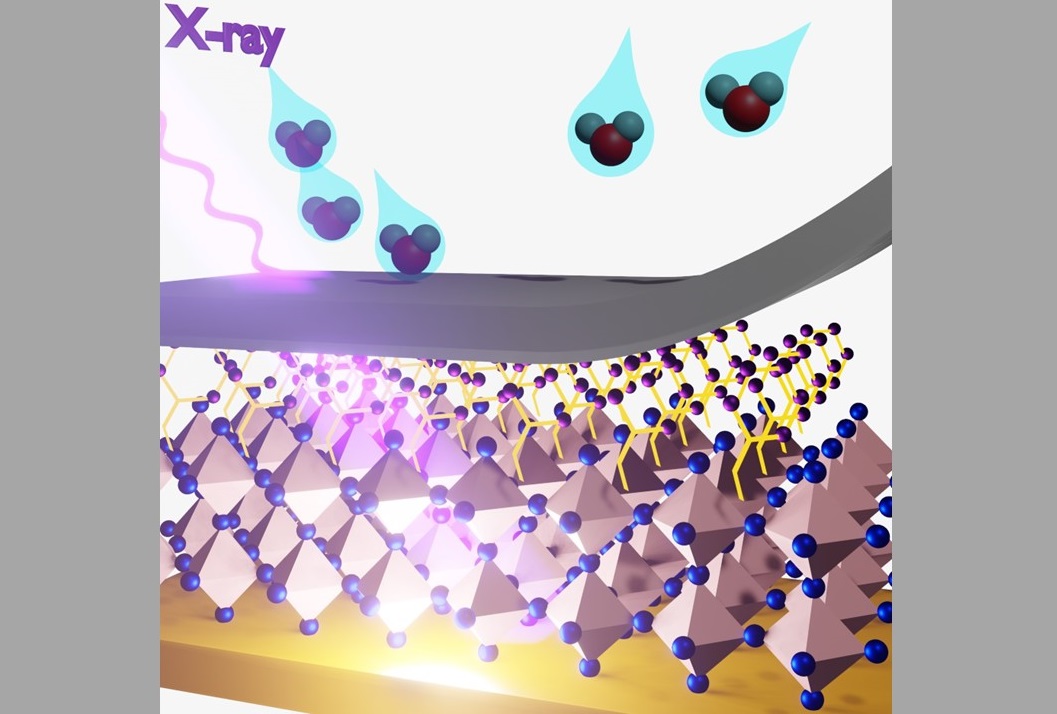
Schematic illustration of the X-ray detector, where a perovskite semiconductor is sandwiched by two charge collection metal electrodes. Once X-ray hits the device, it generates electrical charges that can be collected by the metal electrodes for signal generation. The Los Alamos team developed a hydrophobic interface layer that can expel the water molecules.
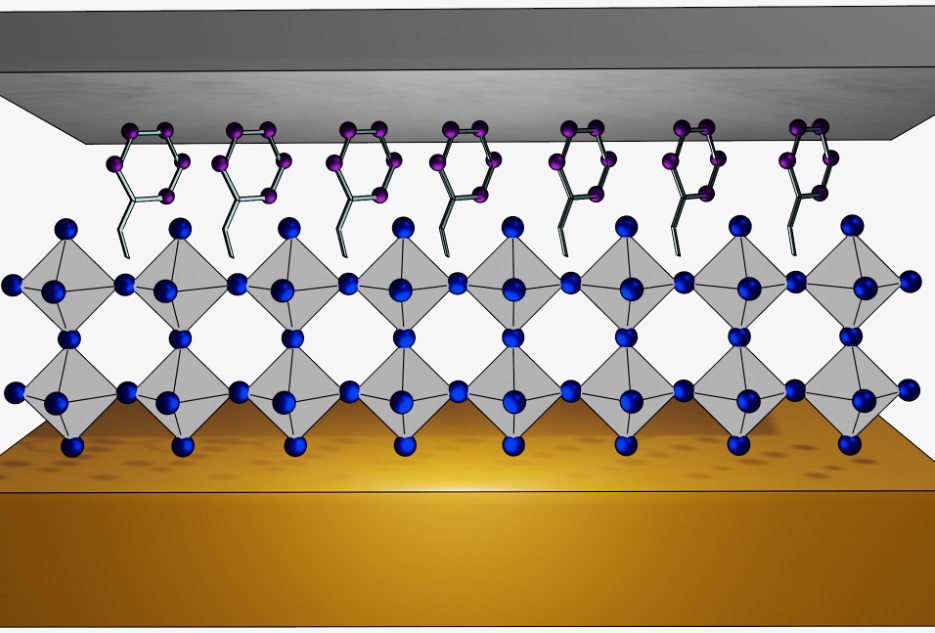
Cross sectional view of the perovskite layer capped with hydrophobic phenylamine molecules for water expelling, between a layer of metal electrodes at top and bottom.
With high dark resistivity and superior photoconductivity, two-dimensional lead-halide perovskites are a promising low-cost semiconductor for radiation photon sensing. Perovskite-based solid-state radiation detectors — semiconductor devices that directly convert a radiation photon to an electrical signal — deliver impressive X-ray sensitivities, surpassing the digital panels sold on the market. Despite the promising performance, their further development has been hampered by an electrical field induced instability.
In research featured on the cover of ACS Energy Letters, a team co-led by Center for Integrated Nanotechnologies scientists Wanyi Nie and Sergei Tretiak identified the cause of the voltage instability and developed a practical strategy to circumvent the problem. Their work provides a mechanistic understanding of the instability and a viable solution toward robust detector development.
To operate the detector at its maximum sensing efficiency, a high electrical field is typically applied to drive the X-ray ionized carriers across the semiconductor. However, perovskite materials are unstable under an electrical field, and a high sensitivity can only last for tens of minutes without protection. The team identified that the voltage-induced instability is directly tied to humidity levels of the two-dimensional perovskite detector’s operational environment. By testing the dark and photocurrent of its photodiode under inert and humid environments, they discovered that a high humidity level promotes a lag in the detector’s photocurrent response when a field is applied and accelerates the device’s failure under a constant electrical field stress.
Building on this observation, the team circumvented the moisture-accelerated device breakdown by growing a hydrophobic molecule over the perovskite surface. In so doing, they found that the device’s operational stability under voltage stress can be enhanced even when operating in a humid environment. The breakdown threshold voltage is pushed to a much higher level, allowing the detector to reach its maximum efficiently without a device failure. Quantum chemical simulations indicate that the hydrophobic molecule forms an atomically compact layer over the perovskite, serving as an efficient moisture barrier.
This work leveraged perovskite crystalline material growth, electronic device fabrication, and optical spectroscopy tools available at the Center for Integrated Nanotechnologies, the Laboratory’s high-performance computing capabilities, and the materials characterization capabilities at the Advanced Photon Source.
Funding and mission
The Los Alamos portion of the work was funded by a Laboratory Directed Research and Development Mission Foundations project and a J. Robert Oppenheimer Distinguished Postdoctoral Fellowship. The research was performed, in part, at the Center for Integrated Nanotechnologies, a DOE Office of Science Basic Energy Sciences user facility operated jointly by Sandia and Los Alamos national laboratories. This work supports the Laboratory’s Energy Security mission and the Materials for the Future capability pillar.
Reference
“Addressing the voltage induced instability problem of perovskite semiconductor detectors,” ACS Energy Letters, 7, 11, 3871 (2022); DOI: 10.1021/acsenergylett.2c02054. Authors: Hsinhan Tsai, Dibyajyoti Ghosh, Cheng-Hung Hou, Sergei Tretiak and Wanyi Nie (Los Alamos National Laboratory); Wyatt Panaccione and Lei Raymond Cao (The Ohio State University); Li-Yun Su and Leeyih Wang (National Taiwan University).
Technical contact: Wanyi Nie (MPA-CINT)
Mapping metastable phases among lanthanide sequioxides
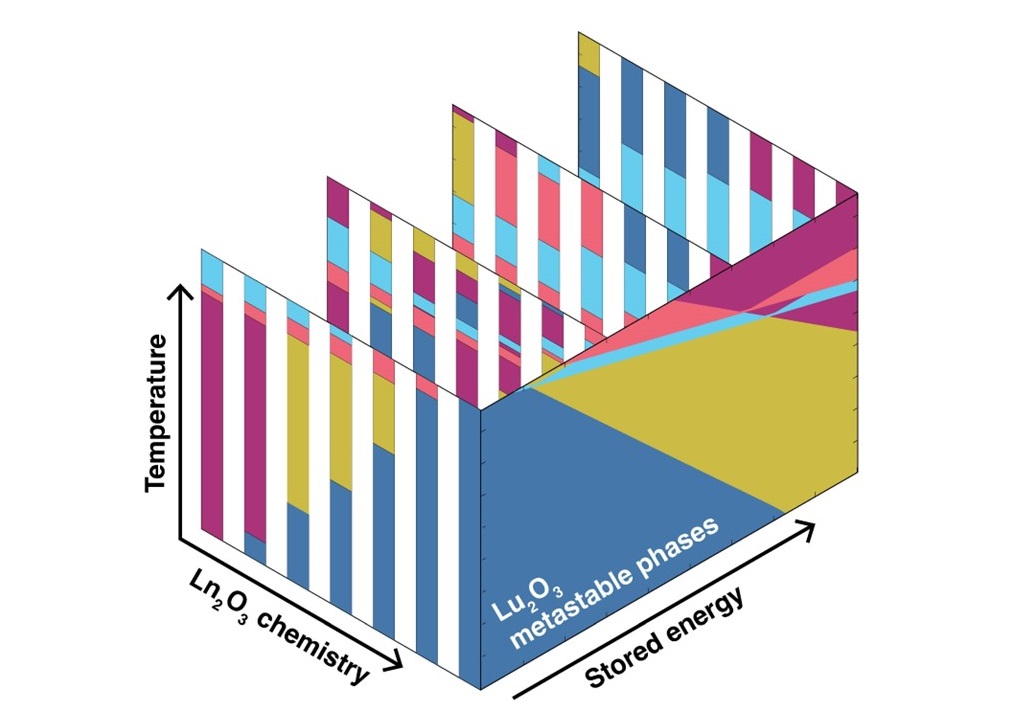
Schematic representation of calculated lanthanide sesquioxides phase diagrams at the ground state (change in stored energy equals zero) and different values of change in stored energy, along with the corresponding metastable phases of lanthanide sesquioxides, as function of change in energy and temperature. The five different phases are represented by the five different colors.
Lanthanide sesquioxides are materials with many different applications, from corrosion resistant materials to solid oxide fuel cells to biomedical applications. The compounds have many different metastable phases — states of matter that are not thermodynamically stable — where the chemistry remains the same, but the crystal structure, or the atomic ordering, changes. To enable efficient and effective fabrication of next-generation technologies, scientists need the ability to predict how to access these different phases. In research described in Materials Advances, a Los Alamos National Laboratory research team linked computer modeling and experimental science to formulate a predictive metastability map, useful for understanding how imparting a certain amount energy on the lanthanide sesquioxide crystal structure can lead to phase changes.
Synthesizing metastable phases currently depends on a trial-and-error approach. The research team sought to understand the amount of stored energy — the metastability threshold — needed to form metastable phases. Lanthanide sesquioxides make an ideal case study to understand metastable phase formation, given their rich polymorphism with various properties and the ease with which they transform to different polymorphs. Using density functional theory to determine the metastability threshold of all relevant phases, the team was able to generate metastable phase diagrams. That work helped understand the formation of metastable phases and predict the specific conditions that can contribute to the formation of particular phases.
The team predicted a series of metastable phase transformations and then showed for the first time, via irradiation, that those phases could be accessed in sequence, correlated with their metastability threshold. The metastability limit for lanthanide sesquioxides, approximately 8 millielectronvolts per atom, was identified, answering questions about the inability to synthesize metastable phases from decomposition of salts or hydrothermal synthesis. Above that threshold, metastable phases cannot be synthesized from decomposition of salts or hydrothermal synthesis. That insight may inform approaches for lanthanide sesquioxide production.
The results offer useful insight into lanthanide sesquioxide metastability that can form the basis for future experimental studies. The same approach can be used to identify the metastable phases that form under non-equilibrium conditions in other materials as well.
The research team notes the development of the metastable phase diagram approach as described in a recent Nature Communications publication by members of Argonne National Laboratory’s Center for Nanoscale Materials.
Funding and mission
The research was supported by the U. S. Department of Energy, Office of Science, Basic Energy Sciences, Materials Sciences and Engineering Division. The work was performed, in part, at the Center for Integrated Nanotechnologies, a DOE Office of Science User Facility, and also used resources of the National Energy Research Scientific Computing Center, a DOE Office of Science User Facility located at Lawrence Berkeley National Laboratory. The work supports the Global Security mission area and the Materials for the Future capability pillar.
Reference
“Predicting and accessing metastable phases,” Materials Advances, 4, 1101 (2023); DOI: 10.1039/D2MA00995A. Authors: V. Kocevski, J.A. Valdez, B.K. Derby, Y.Q. Wang, G. Pilania and B.P. Uberuaga (Los Alamos National Laboratory).
See also, “Machine learning the metastable phase diagram of covalently bonded carbon,” Nature Communications, 13 (2022); DOI: 10.1038/s41467-022-30820-8. Authors: Srilok Srinivasan, Rohit Batra, Duan Luo, Troy Loeffler, Sukriti Manna, Henry Chan, Jianguo Wen, Pierre Darancet and Subramanian K.R.S. Sankaranarayanan (Argonne National Laboratory); and Liuxiang Yang and Wenge Yang (Center for High Pressure Science and Technology Advanced Research, China).
Technical contacts: Benjamin Derby (MPA-CINT) and Blas Uberuaga (MST-8)
Materials Science and Technology
Novel technique holds potential for developing viable anode-free solid-state batteries
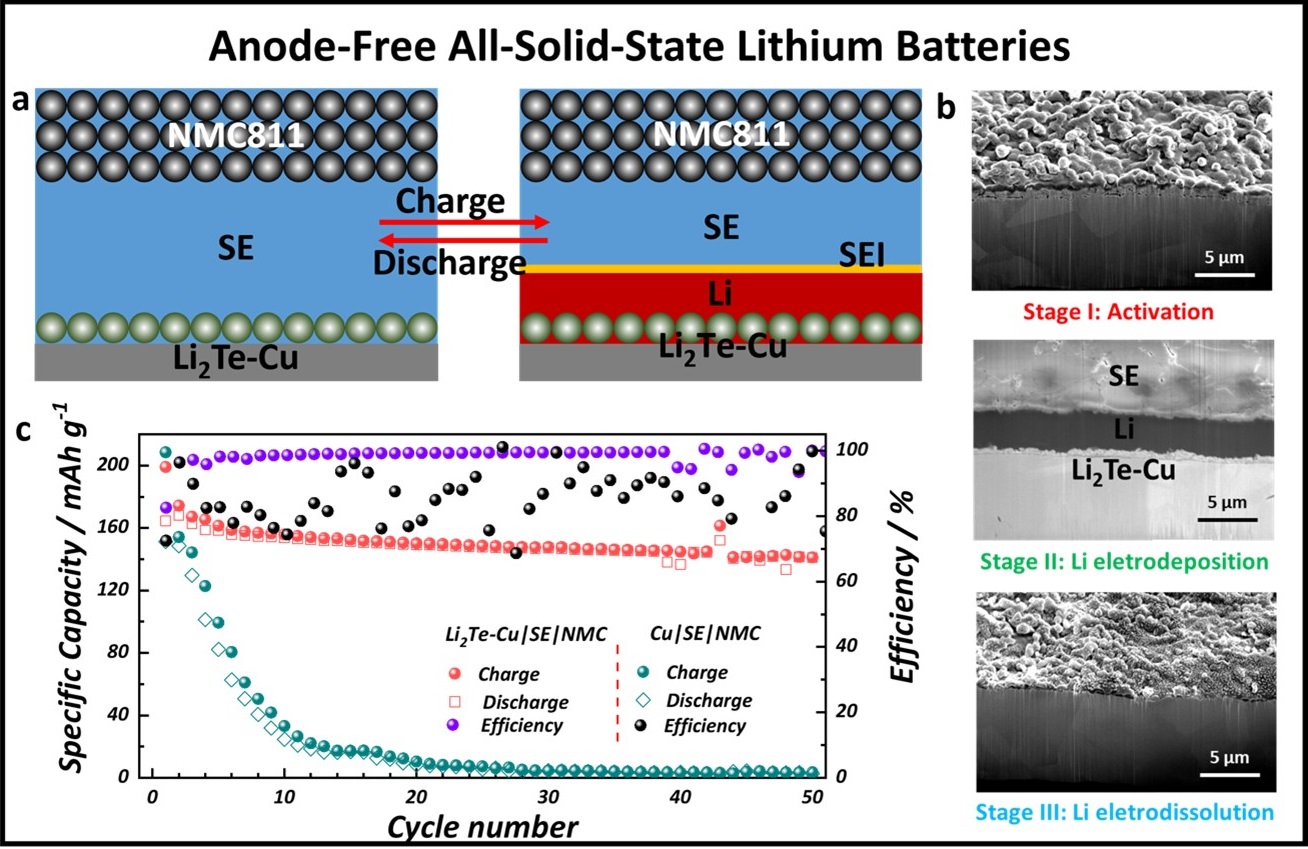
The figure shows a) Schematic diagram of the working principle in an anode-free all-solid-state battery. Dendrite formation is suppressed by first forming a lithiophilic Li2Te-Cu current collector which promotes uniform wetting of lithium metal. b) Cryogenic focused ion beam (cryo-FIB) reveals uniform lithium deposition and no dendrite formation. c) Anode-free ASSB cycling performance of the lithiophilic Li2Te-Cu current collector showing much better performance than when a bare copper current collector is used.
Anode-free all-solid-state batteries hold potential as promising energy storage devices due to their high energy capacity and safety features. In particular, sulfide solid-state electrolytes used in these batteries display highly promising ionic conductivities and offer advantages over lithium metal-based versions. However, sulfide solid-state electrolytes still suffer from several major shortcomings. One key issue is the reactivity between the sulfides and both electrodes, which leads to an impedance rise associated with the formation of a mixed conducting interphase.
Using the unique capabilities available at the Center for Integrated Nanotechnologies, a team of researchers achieved a stable anode-free all-solid-state battery with a sulfide-based solid-electrolyte by tuning the wetting properties of lithium metal on the copper current-collector. Lithiophilic 1 micrometer Li2Te is synthesized by exposing the collector to tellurium vapor, followed by in situ lithium activation during the first charge, which promotes uniform wetting of lithium metal, and suppresses dendrite formation. Their work paves the way for viable anode-free all-solid-state batteries that deliver significantly higher specific energies and cost less than all-solid-state batteries with excess lithium sources. Advanced Materials published the work.
Cryogenic focused ion beam (cryo-FIB) milling and cryo-SEM analysis of the solid-state electrolyte-electrode interface was performed at the Center for Integrated Nanotechnologies using the Scios 2 dual beam focused ion beam scanning electron microscope with a Leica VCT cryogenic stage and X-ray energy dispersive spectroscopy detector. To preserve the structural integrity of the beam-sensitive lithium-based materials and to reduce artificial inclusion, the sample was cooled to minus 150 degrees Celsius.
Funding and mission
This work is funded by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences; the Center for Integrated Nanotechnologies is an Office of Science User Facility operated for the DOE Office of Science. The research supports the Laboratory Energy Security mission and the Materials for the Future capability pillar.
Reference
“Stable anode-free all-solid-state lithium battery through tuned metal wetting on the copper current collector,” Advanced Materials, 2206762 (2022); DOI: 10.1002/adma.202206762. Authors: Yixian Wang, Yijie Liu, Mai Nguyen, Jaeyoung Cho, Naman Katyal, Hongchang Hao, Ruyi Fang, Nan Wu, Pengcheng Liu, Graeme Henkelman, David Mitlin (The University of Texas at Austin); Bairav S. Vishnugopi and Partha P. Mukherjee (Purdue University); Jagjit Nanda (SLAC National Laboratory); and John Watt (Los Alamos National Laboratory).
Technical contact: John Watt (MPA-CINT)
Research sheds light on mechanisms impacting fuel cell durability
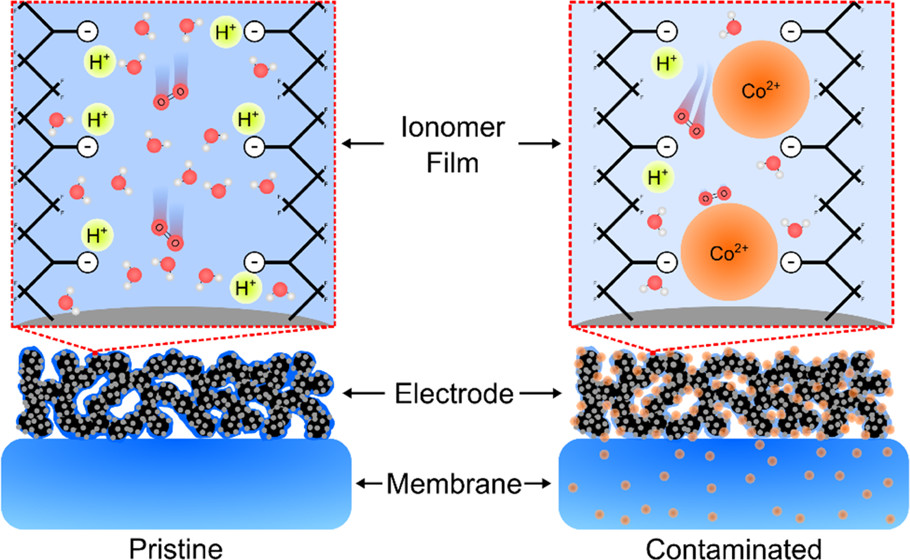
Left: pristine membranes and electrodes enable rapid transport of protons and oxygen molecules. Right: cobalt-contaminated membranes and electrodes suffer from slow transport of protons and oxygen. Image published in ACS Applied Materials and Interfaces, used via Creative Commons license.
With the transportation sector a major contributor to global carbon dioxide emissions, proton exchange membrane fuel cells (PEMFCs) offer a promising alternative to internal combustion engines due to their ability to produce electricity on demand without any local carbon dioxide emissions. However, despite significant technical improvements for PEMFCs, cost and durability remain major barriers to their wider adoption.
Metal alloy catalysts (e.g., platinum-cobalt) are widely used in PEMFC fuel cells to improve the electrochemical energy conversion process yet they diminish the cell’s durability. Leaching of the cobalt alloying element contaminates the ionomer/membrane, leading to poor durability. The mechanisms by which this contamination affects fuel cell performance remain poorly understood.
In work highlighted on the cover of ACS Applied Materials and Interfaces, a team at Los Alamos report on the first-of-its-kind mechanistic explanation for cation effects and strategies for mitigating these undesired effects when using alloy catalysts. Their findings demonstrate a powerful platform for accurately investigating cation contamination effects in PEMFC electrodes, which can support the design of methods to mitigate undesired cobalt leaching effects in next-generation PEMFCs, as well as other emerging electrochemical devices that may suffer from cation contamination.
The researchers systematically coupled electrochemical testing results and membrane conductivity/water uptake measurements and impedance modeling to pinpoint where and how performance losses occur. The team identified three main factors: First, roughly 44% of cobalt exchange of the ionomer can be tolerated in the electrode. Second, loss in performance is predominantly induced by oxygen and proton transport losses, and finally cobalt preferentially resides in the electrode under wet operating conditions.
The work leveraged Los Alamos capabilities in fuel cell membrane electrode assembly fabrication and testing. Advanced X-ray fluorescence mapping capabilities were used to measure the amount and distribution of cobalt ions in the membrane electrode assembly.
Funding and mission
This work was supported by the DOE Office of Energy Efficiency and Renewable Energy’s Hydrogen and Fuel Cell Technologies Office through the Million Mile Fuel Cell Truck consortia, Los Alamos’s Laboratory Directed Research and Development program and the Natural Sciences and Engineering Research Council of Canada. The work supports the Energy Security mission area and the Materials for the Future capability pillar.
Reference
“Toward a comprehensive understanding of cation effect in proton exchange membrane fuel cells,” ACS Applied Materials and Interfaces, 14, 31, 35555 (2022); DOI: 10.1021/acsami.2c07085. Authors: ChungHyuk Lee, Siddharth Komini Babu, Jacob S. Spendelow, Rangachary Mukundan and Rod L. Borup (Los Alamos National Laboratory); Xiaohua Wang, Jui-Kun Peng and Rajesh K. Ahluwalia (Argonne National Laboratory); and Adlai Katzenberg and Ahmet Kusoglu (Lawrence Berkeley National Laboratory).
Technical contact: Rod L. Borup (MPA-11)
Materials-by-design approach reveals lowered work function for lanthanum hexaboride
Experimentalist Hisato Yamaguchi, left, and Theoretical division theorist Gaoxue Wang, right, hold an atom structure model.
In work recently published in Applied Physics Letters and selected as an Editor’s Pick, a research team led by Los Alamos National Laboratory scientists demonstrated the first ever lowering of work function for lanthanum hexaboride, a widely used electron emissive material, by coating its surface with a two-dimensional, atomically thin layer of hexagonal boron nitride. Work function is the minimum energy required for electrons to escape from a material surface into a vacuum, thus an important property associated with the cathode technology in devices from photosensors and microscopes to accelerators. For photocathodes, work function determines quantum efficiency, the rate of free electrons generated per incident photons. Exemplifying a “materials by design” approach that combines theoretical and experimental research, the work demonstrates an efficient way to tune materials properties.
The team used photoemission electron microscopy (PEEM) and thermionic emission electron microscopy (TEEM) to discover that when coated with hexagonal boron nitride, a single crystal of lanthanum hexaboride has lower work function compared to non-coated (bare) and graphene-coated regions. A 0.4 electronvolt decrease in work function quantitatively supported the broad and uniformly brighter image of the hexagonal boron nitride region revealed by PEEM. The team also found, through TEEM, that unlike the bare and non-coated regions, the hexagonal boron nitride region exhibited thermionic emission at 905 degrees Celsius. Density functional theory calculations qualitatively supported the work function decrease in the hexagonal boron nitride-coated lanthanum hexaboride.
By adding an oxide layer in the calculations, the team was able to improve consistency between the theoretical inputs and experimental results. Synchrotron-radiation X-ray photoelectron spectroscopy confirmed the presence of an oxide layer on the lanthanum hexaboride. The degree of work function modification on hexagonal boron nitride and graphene-coated oxidized surfaces proved to be smaller than on the clean surfaces. That result indicates that the oxide layer reduces the change transfers between the lanthanum hexaboride and the two-dimensional materials.
Theoretical inputs helped steer the experimental research, predicting that hexagonal boron nitride would lower the work function, whereas a similarly atomically thin material, graphene, would not. Without the theoretical inputs, experimentalists in the project did not foresee a possibility that an atomically thin material coating would lower the work function of electron emissive materials like lanthanum hexaboride. Even if experimentalists have thought of this concept, finding the right atomically thin materials out of hundreds to thousands of material choice is unrealistic, so the ability of theoretical calculations to pinpoint a promising candidate material with a desired effect was important.
Funding and mission
The work was supported by the U.S. Department of Energy Office of Science, U.S.-Japan Science and Technology Cooperation Program in High Energy Physics. The work was also supported, in part, by the Laboratory Directed Research and Development program at Los Alamos. Studies were performed, in part, at the Center for Integrated Nanotechnologies, an Office of Science User Facility, operated for the U.S. Department of Energy Office of Science. The work supports the Global Security mission area and the Materials for the Future capability pillar.
Reference
“Work function lowering of LaB6 by monolayer hexagonal boron nitride coating for improved photo- and thermionic-cathodes,” Applied Physics Letters, 122, 14 (2023); DOI: 10.1063/5.0142591. Authors: Hisato Yamaguchi, Gaoxue Wang, Michael T. Pettes and Nathan A. Moody (Los Alamos National Laboratory); Ryunosuke Yusa and Tadashi Abukawa (Tohoku University); Shuichi Ogawa (Nihon University); Fangze Liu (Beijing Institute of Technology); and Yasutaka Tsuda and Akitaka Yoshigoe (Japan Atomic Energy Agency).
Technical contact: Hisato Yamaguchi (AOT-AE)
Theoretical
Kagome qubit spin ice realized in quantum annealing processor
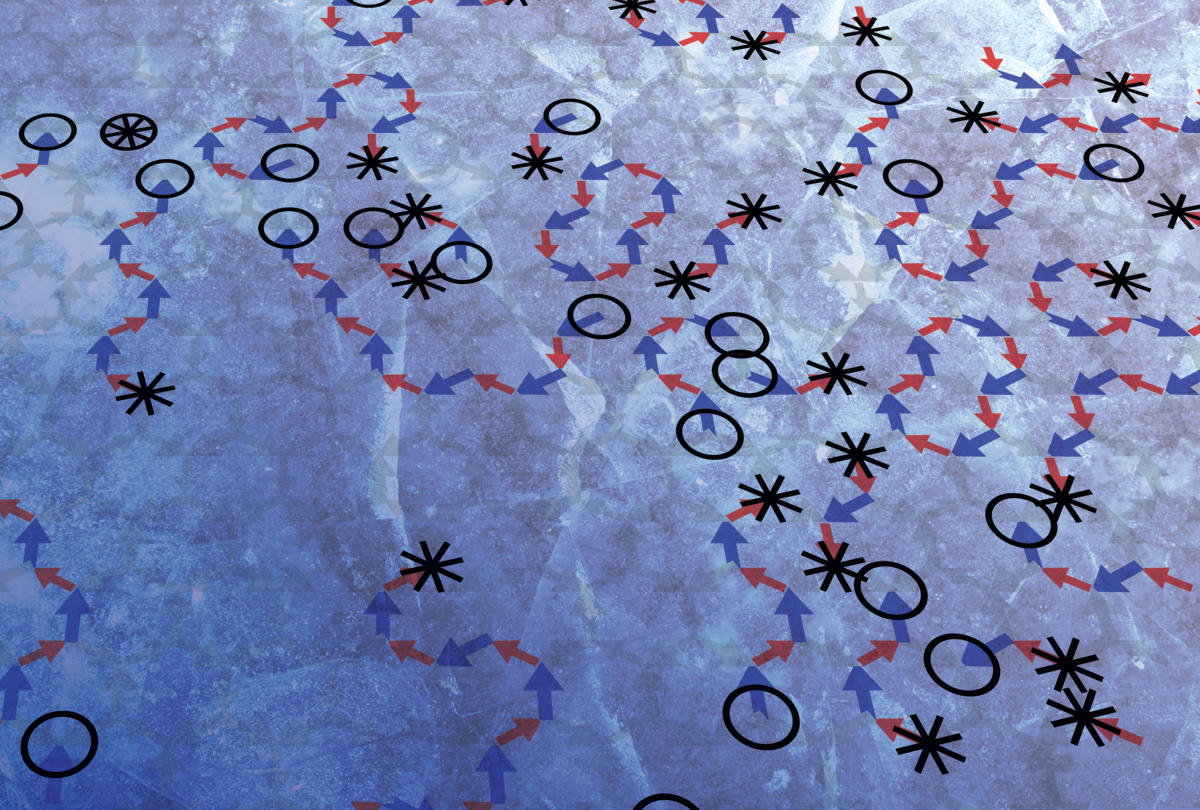
Fractionalized excitations move by flipping qubits in the Kagome Ice-II disordered manifold.
Materials hosting spins arranged in a triangular geometry such as the kagome lattice are one of the cornerstones of frustrated magnetic materials. For decades, experimental studies of kagome spin liquids, in which magnetic moments remain disordered at all temperatures, have been restricted to those found in natural materials. Recently, nanomagnets have been artificially created to reveal novel exotic classical behavior. Now, a team of scientists from the Theoretical division at Los Alamos National Laboratory, in collaboration with researchers from D-Wave, has used a quantum annealing processor to realize a controllable quantum version of a kagome spin lattice for the first time.
D-Wave’s quantum platform is composed of interconnected qubits able to find a system's lowest energy state by slowly reducing quantum fluctuations. The authors embedded a kagome lattice in a quantum annealer so that which qubits sit at the triangle vertices in a frustrated configuration. This frustration is what makes the system interesting: Because each qubit’s magnetic moment is not “comfortable” in any possible orientation, the states of the qubit remain disordered.
The realization in 2,742 superconducting qubits of this exotic behavior of magnetic moments is described in Nature Communications and is presented as an opportunity to explore field-induced spin-liquid phases. Including a magnetic field in the experiments allowed the team to explore different phases of the magnetic moment arrangement. Similar to what happens when liquid water turns into vapor when heat is applied, the magnetic field induces a kinetic crossover between the Ice-I phase and an unconventional field-induced Ice-II phase.
The team showed that the special geometry of the kagome lattice, together with the interaction constraints imposed on the qubits and the selective ordering that the field enforces over the magnetic moment distribution, allow a set of spins to group, form “spinon” pseudoparticles that are created and annihilated in pairs of opposite topological charge, and move around the lattice. A large-scale and fully functional control of pseudoparticles is the holy grail of quantum information sciences that search to handle many of them over time, limiting the effect of external perturbations that would destroy them. The research team showed that only in a phase of the material, the so-called Ice-II phase, the kinetics is topologically protected leading to charged spinons that behave like particles/antiparticles. They further demonstrated a kinetics activated by quantum rather than merely thermal fluctuations, which the D-Wave processor can generate.
The team’s results highlight the capabilities of quantum annealers for implementing exotic phases of frustrated spin liquids that are open to design, and activating them via controllable quantum fluctuations in quantum annealing processors. As the coherence of quantum annealer increases, continued research in this area will advance the study of new states of quantum matter and holds significant implications for future proposals on quantum computing.
Funding and mission
The work supports the Global Security mission area and the Information, Science and Technology capability pillar.
Reference
“Kagome qubit ice,” Nature Communications, 14, 1105 (2023); DOI: 10.1038/s41467-023-36760-1. Authors: Alejandro Lopez-Bezanilla and Cristiano Nisoli (Los Alamos National Laboratory); Jack Raymond, Kelly Boothby and Andrew D. King (D-Wave Quantum Inc.); and Juan Carrasquilla (University of Toronto).
Technical contact: Cristiano Nisoli (T-4)